1. The Nature of Gums and Phosphatides
Crude oil obtained by screw pressing and solvent extraction of oilseeds will throw a deposit of so-called gums on storage. The chemical nature of these gums has been difficult to determine. They contain nitrogen and sugar and can start fermenting so they were at one stage thought to consist of glycolipids and proteins. Now we know that these gums consist mainly of phosphatides but also contain entrained oil and meal particles. They are formed when the oil absorbs water that causes some of the phosphatides to become hydrated and thereby oil-insoluble. Accordingly, hydrating the gums and removing the hydrated gums from the oil before storing the oil can prevent the formation of a gum deposit. This treatment is called water degumming. It is never applied to fruit oils like olive oil and palm oil since these oils have already been in contact with water during their production.
Water degumming is the oldest degumming treatment and also forms the basis of the production of commercial lecithin. I use the term 'commercial lecithin' here to make a distinction from the use of the word 'lecithin' as the trivial name for the compound phosphatidylcholine (PC). Similarly, phosphatidylethanolamine (PE) has the trivial name 'kephalin'. Since the water degumming process involves more water than when crude oil is allowed to absorb moisture from the atmosphere, the gums resulting from the water degumming process also remove hydrophilic substances such as sugars from the oil.
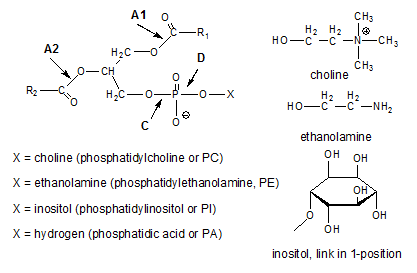
Lecithin as obtained by drying the gums resulting from the water degumming process contains a mixture of different phosphatides. The structural formulae of the main phosphatides present in lecithin are shown in Figure 1 (further information on phosphatides is available here...).
Figure 1. Chemical structure of most common phosphatides and indication of bonds that are hydrolysed by various phospholipase enzymes.
Table 1 gives the phosphatide composition of the phosphatide fraction in lecithins obtained from different oils.
| Table 1. Composition (wt %) of phosphatides of various lecithins, adapted from [1] | |||
| Phosphatide | Soyabean | Sunflower seed | Rapeseed |
| PC | 32 | 34 | 37 |
| PE | 23 | 17 | 20 |
| PI | 21 | 30 | 22 |
| PA | 8 | 6 | 8 |
| Others | 15 | 13 | 13 |
Please keep in mind that Table 1 refers to lecithins, the mixture of phosphatides that has been obtained by degumming crude oil with water. Since this water degumming process does not remove all phosphatides from the oil, Table 1 does not reflect the composition of the phosphatides present in the crude oil itself.
Just as a triglyceride oil is a mixture of triacylglycerols with different fatty acids, each phosphatide is also a mixture of different compounds. These compounds differ in their fatty acid composition and isomerically, in their location on the glycerol backbone. In general, the fatty acid composition of the phosphatides reflects the fatty acid composition of the oil in which these phosphatides occur but it tends to have a higher palmitic acid content and a lower oleic acid content than the oil as illustrated by Table 2.
| Table 2. Fatty acid compositions of vegetable lecithins and oils. Adapted from [1] and [2] | ||||||
| Fatty acid | Soya bean | Sunflower seed | Rapeseed | |||
|---|---|---|---|---|---|---|
| Lecithin | Oil | Lecithin | Oil | Lecithin | Oil | |
| 16 | 11 | 11 | 7 | 7 | 4 | |
| 18:0 | 4 | 4 | 4 | 5 | 1 | 2 |
| 18:1 | 17 | 23 | 18 | 29 | 56 | 61 |
| 18:2 | 55 | 54 | 63 | 58 | 25 | 22 |
| 18:3 | 7 | 8 | 0< | 0 | 6 | 10 |
| Others | 1 | 0 | 4 | 1 | 5 | 1 |
The above table contains the data required for arriving at a conversion factor that permits the amount of phosphatides present in the oil to be calculated from its phosphorus content. For the oils represented in Table 2, this factor equals about 25 to 26 [3]. In other words, oil containing say 200 ppm of phosphorus contains about 0.5 wt% phosphatides.
On the other hand, the literature often uses a factor of 31.5 [3] or thereabouts to arrive at the acetone-insoluble component of the lecithin. This difference stems from the fact that the acetone-insoluble component of lecithin also comprises glycolipids and sugars. The factor of 31.5 is therefore very much an empirical value. It should only be used for oils that have not yet been water-degummed since on water degumming, sugars are removed. For water-degummed oils, which contain alkaline earth salts of PA and lysophosphatidic acid (LPA) and some PE and lysophosphatidylethanolamine (LPE) and do not contain any more sugars, a factor of 23 to 24 should be used to convert phosphorus to phosphatides.
2. Hydratability of Phosphatides
The extent to which a phosphatide present in the crude oil is removed during water degumming depends on its hydrophilicity. Phosphatidylinositol has five free hydroxyl groups on the inositol moiety that make PI strongly hydrophilic. Consequently, PI present in crude oil will be hydrated during the water degumming treatment and the PI content of properly water-degummed oil is negligible. Similarly, the positive charge of the trimethylamino group in phosphatidylcholine makes this phosphatide hydrophilic. This hydrophilicity does not depend on the pH of the water used to degum the oil since even at pH > 5, when the phosphate group in the PC is dissociated and therefore carries a negative charge, it does not form an internal salt with the quaternary amino group for steric reasons. Consequently, the positive quaternary amino group remains isolated at all pH values and causes PC to be hydrophilic at all pH values.
Table 3 shows what charges the various phosphatides carry at which pH.
| Table 3. Charges of phosphatides as a function of pH | |||||
| pH | PC | PE | PI | PA | Ca-PA |
| 2 | + | + | 0 | 0 | 0 |
| 3 | (+) | (+) | (0) | (0) | 0 |
| 4 | (±) | (±) | (-) | (-) | 0 |
| 5-7 | ± | ± | - | - | 0 |
| 8-9 | ± | ± | - | (2-) | 0 |
| >10 | ± | - | - | 2- | 0 |
Some charges in Table 3 have been put between parentheses. They indicate a transition between the value at lower pH and the value at higher pH. So according to Table 3, almost all phosphatidylethanolamine (PE) molecules have a positive charge at pH = 2. This charge causes these molecules to be hydrophilic so at that pH, PE is hydratable. When the pH is increased, more and more phosphate groups dissociate and so a zwitterion (indicated by ±) is formed in which the positive amino group forms an internal salt with the negative phosphate group. The positive and negative charges are so close together that the hydrophilicity of this zwitterion is quite weak and on water degumming, the hydration of PE is incomplete. Accordingly, water-degummed oil still contains some PE.
Now we come to phosphatidic acid (PA). In an acid environment, the hydroxyl groups of its phosphate moiety will not dissociate since the pKa value of the first hydroxyl group equals 2.7-3.8 [4]. Consequently, PA will be poorly hydratable and remain in the oil when this is brought into contact with acid water. When the pH of this water is raised to 5, most of the PA will be dissociated so that the molecule has a negative charge giving it a hydrophilicity that makes it hydratable. Accordingly lecithin contains some PA as illustrated by Table 1. When the pH of the water is raised even further, the second hydroxyl will also dissociate since its pKa is 7.9-8.6 [4], whereby the actual value depends on what other salts are present in the water.
But what about the calcium salt of PA? According to the column on the far right in Table 3, this remains without charge at all pH values because the divalent calcium forms a salt with the two dissociated hydroxyl groups of the phosphate moiety. That is the reason that alkaline earth salts of PA remain in the oil when it is degummed with water. They are the main constituents of the nonhydratable phosphatides (NHP). However, when the oil is alkali refined, these salts are removed. Two possible mechanisms have been shown in Figure 2:
Figure 2. Calcium phosphatidate at high pH.
In the lefthand structure in Figure 2, a hydroxyl ion has been linked to the Ca2+ ion so that it only has a single positive charge and the salt itself has a negative charge making it hydratable. In the righthand structure, the hydroxyl ion has been linked to the phosphorus of the phosphate moiety so that the calcium retains its 2+ charge. Because of the addition of a negative hydroxyl group the salt itself becomes negatively charged and thus hydratable.
PA moieties present in crude oil are generally considered to originate from the hydrolysis of phosphatides such as PC, PE and PI. This hydrolysis is most likely catalysed by phospholipase D (see Fig. 1). Phospholipase A1 and A2 on the other hand lead to the formation of lysophosphatides by hydrolysing one of the ester bonds between a fatty acid and the glycerol moiety in the phosphatide. What about the hydratability of these lysophosphatides? Their free hydroxyl group is more hydrophilic than the original fatty acid ester, but does this make them hydratable when the parent compound is nonhydratable?
The answer to this question is not straightforward. According to [5], the nonhydratable phosphatides (NHP) comprise lysophosphatidic acid (LPA) and lysophosphatidylethanolamine (LPE), indicating that lysophosphatides are not completely hydratable. According to [6], enzymatic hydrolysis of the NHP present in the oil phase using phospholipase A1 and phospholipase A2 led to lyso-compounds that were only detected in the aqueous phase indicating the hydrolysis of NHP causes the resulting lyso-compounds to migrate to the aqueous phase.
In the case of partial glycerides, 1,3-diglycerides are more stable than 1,2-diglycerides. Similarly, 1-/3-(α)-monoglycerides are more stable than 2-(β)-monoglycerides so there is a preference for the fatty acid to be bound to the 1- and 3-positions. It is therefore to be expected that 1-acyl lysophosphatides are more stable than 2-acyl lysophosphatides and that the 2-acyl lysophosphatides formed by the action of the phospholipase A1 will isomerise to 1-acyl lysophosphatides. These have a fatty acid linked to a terminal carbon atom of the glycerol moiety and will therefore be prone to phospholipase A1-catalysed hydrolysis. This will lead to formation of a glycerophosphate and indeed glycerophosphates have been observed in the aqueous phase of oils treated with phospholipase A1 [7]; their concentrations were about equal to those of the lysophospholipids. However, in [6], the use of Lecitase® 10L (a phospholipase A2) led to lower concentrations of lysophosphatides in the aqueous phase than when a phospholipase A1 was used. This might indicate a higher stability of the 2-acyl lysophosphatides in comparison with their 1-isomers.
3. Kinetics of Degumming Processes
The discussion of the hydratability of phosphatides indicates that their molecular structure determines whether they remain in the oil phase or move to the water phase when the oil containing them is contacted with water. They do not divide themselves over the two phases like isopropanol would do when added to a mixture of hexane and water. PE may be an exception in that the literature [8] suggests that PE is only removed on water degumming if other phosphatides with which the PE can form mixed micelles are present. PC is hydratable so there should be no residual PC in water-degummed oil. However, the analyses of quite a few samples of water degummed oil show some PC to be present. How come?
The reason lies in the kinetics of the degumming process. The samples still containing some PC do not represent the equilibrium situation and have not been given enough time to reach equilibrium. So when the partially degummed samples are again subjected to a water degumming treatment, their PC content will drop to the low level commensurate with the hydratability of PC. However, time is not the only factor involved. The interface between the oil and the water and the diffusion distance towards this interface are other factors affecting the hydration kinetics.
In the literature [9], relative rates of hydration have been reported and the values are shown in Table 4.
| Table 4. Relative rate of hydration of various phospholipids | |||
| Phospholipid | Relative rate of hydration | Phospholipid | Relative rate of hydration |
|---|---|---|---|
| PC | 100 | PE (calcium salt) | 0.9 |
| PI | 44 | PA | 8.5 |
| PI (calcium salt) | 24 | PA (calcium salt) | 0.6 |
| PE | 16 | Phytosphingolipid (calcium salt | 8.5 |
The values in this table have been quoted over and over again despite the fact that the article containing this table [9] and its table raise many questions. It mentions calcium salts of PI and PE but does not indicate their molecular structures. However, my main problem with Table 4 is that the article [9] does not indicate at all how these relative rates have been determined. Moreover, the fact that each phosphatide has a rate of hydration that is larger than zero implies that with some patience, water degumming should lead to complete removal of all phosphatides from the oil and that is not what is observed.
On the other hand, my doubts about the relative rates of hydration tabulated above do not mean that I do not recognise the existence of rate differences. When water is used as degumming agent, every phosphatide molecule reaching the oil/water interface encounters this agent. Yet, when an acid that is dissolved in this water has to interact with the phosphatides reaching this interface, most phosphatides will encounter just water and only a few will meet with the acid and react. This has important practical consequences.
In the water degumming process, the water has to be dispersed in the oil but the degree of dispersion is not very critical. A reasonable dispersion will already provide such an oil/water interface that hydratable phosphatides are hydrated and move into the water phase. For the acid to react with the nonhydratable phosphatides and decompose the NHP, a much finer dispersion is required because both reagents are diluted. A very fine dispersion is especially needed when the reaction has to be almost completed and a very low residual phosphatide content has to be reached. Moreover, the situation is aggravated because the water/oil dispersion is not stable. Aqueous acid droplets will coalesce, the interface will decrease, diffusion distances will increase and all this will slow down the reaction. Accordingly, the dispersion has to be so fine that the reaction between the acid and the NHP is almost instantaneous or at least almost completed within a minute.
These requirements are well illustrated by comparing the SOFT degumming process [10] and the Complete degumming process [11]. Both processes employ a salt of ethylene diamine tetraacetic acid (EDTA) as chelating agent to remove metal ions such as calcium ions from the NHP but they differ in that the process according to [10] employs an emulsifier to retard coalescence of the aqueous phase droplets and thus prolongs the reaction between the EDTA and the NHP. The process according to [11], on the other hand, starts with a very fine dispersion of the aqueous solution of the chelating agent in the oil to be degummed and thereby achieves an almost complete reaction between the EDTA and the NHP before coalescence starts to slow down the rate of reaction. The importance of a fine dispersion in degumming had already been pointed out by Mag and Reid [12] and Dijkstra and Van Opstal [13].
For the enzymatic degumming processes the dispersion of the aqueous phase is even more important since on a molar basis, the enzyme concentration is much lower than the concentrations of acid degumming agents and steric requirements lead to a lower Arrhenius factor for enzymatic reactions. In my recent review of enzymatic degumming [14], I referred to [15] which presentation shows that for a given degree of dispersion, the rate of the enzymatic reaction with NHP is an order of magnitude lower than the rate with highly diluted citric acid.
This degree of dispersion was maintained by circulating the contents of the laboratory reaction vessel three times per minute by means of a Silverson mixer. Doing something like that on industrial scale is impossible, which implies that in industrial degumming processes, enzymes do not interact with the NHP present in the oil phase. For the enzymatic degumming process to arrive at low residual phosphorus levels, it has to be preceded by a treatment with a finely dispersed acid that converts the NHP to PA. By raising the pH, this PA moves into the aqueous phase and once there it has become accessible to enzymes.
Abbreviations: PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PA, phosphatidic acid; LPE, lysophosphatidylethanolamine; LPA, lysophosphatidic acid; NHP, non-hydratable phosphatide.
References
https://lipidlibrary.aocs.org/edible-oil-processing/introduction-to-degumming
- Nieuwenhuyzen, W. van and Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol., 110, 472-486 (2008) (DOI: 10.1002/ejlt.200800041).
- Gunstone, F.D. and Harwood, J.L. Occurrence and characterisation of oils and fats. In: The Lipid Handbook (3rd edition), pp.37-142 (F.D. Gunstone, J.L. Harwood, and A.J. Dijkstra (eds.), Taylor & Francis Group, LLC, Boca Raton, FL) (2007).
- Pardun, H. Neuberechnung der zur Bestimmung des Phosphatidgehalts benötigten Umrechnungfaktoren. Fette Seifen Anstrichm., 83, 240-242 (1981).
- Abramson, M.B., Katzman, R., Wilson, C.E. and Gregor, H.P. Ionic properties of aqueous dispersions of phosphatidic acid. J. Biol. Chem., 239, 4066-4072 (1964).
- Dijkstra, A.J. and Van Opstal, M. The total degumming process. J. Am. Oil Chem. Soc., 66, 1002-1009 (1989).
- Clausen, K. Enzymatic oil-degumming by a novel microbial phospholipase. Eur. J. Lipid Sci. Technol., 103, 333-340 (2001).
- Yang, B., Zhou, R., Yang, J.-G., Wang, Y.-H. and Wang, W.-F. Insight into the enzymatic degumming process of soybean oil. J. Am. Oil Chem. Soc., 85, 421-425 (2008) (DOI: 10.1007/s11746-008-1225-y).
- Kanamoto, R., Wada, Y., Miyajimi, G. and Kito, M. Phospholipid-phospholipid interaction in soybean oil. J. Am. Oil Chem. Soc., 58, 1050-1053 (1981).
- Sen Gupta, A.K. Neuere Entwicklungen auf dem Gebiet der Raffination der Speiseöle. Fette Seifen Anstrichm., 88, 79-86 (1986).
- Jamil, S., Dufour, J.-P.G. and Deffense, E.M.J. (Fractionnement Tirtiaux S.A.), Process for degumming a fatty substance and fatty substance thus obtained, US Patent 6,015,915 (2000).
- Deffense, E.M.J. Method for eliminating metals from fatty substances and gums associated with said metals, US Patent 6,407,271 (2002).
- Mag, T.K. and Reid, M.P. (Canada Packers Limited), Continuous process for contacting of triglyceride oils with an acid, US Patent 4,240,972 (1980).
- Dijkstra, A.J. and Van Opstal, M. (N.V. Vandemoortele International), Process for producing degummed vegetable oils and gums of high phosphatidic acid content, European Patent 0 195 991 (1986).
- Dijkstra, A.J. Enzymatic degumming. Eur. J. Lipid Sci. Technol., 112, 1178-1180 (2010) (DOI: 10.1002/ejlt.201000320).
- Clausen, K., Nielsen, P.M., Andreasen, L.L., Petterson, H.F. and Borch, K. A new microbial phospholipase for degumming of vegetable oil. Paper presented at the 93rd AOCS Annual Meeting & Expo, Montréal (2002).
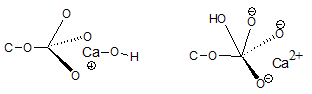
No comments:
Post a Comment